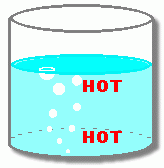
What's going on? Why does the noise and vibration happen for a little while, and then stop? 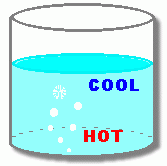 As the water is heated, the bottom layer of water gets hot enough (100° C) to begin turning some of the water from a liquid to a gas. This gas is called water vapour, and it forms bubbles in the liquid. Since these gas bubbles are less dense than the water around them, they begin to rise. At this point, however, the top layer of the water is still cool. When the water vapour bubbles rise into this cool layer, they are cooled enough so that the vapour condenses back into a liquid. The bubbles collapse.  Look at the close-up. As the vapour turns back into a liquid, with less volume, the bubbles collapse. This leaves little holes in the water, wherever there was a bubble. The surrounding liquid rushes in to fill the empty spaces where the bubbles were. This makes 'popping' noises. When enough of these bubbles are popping, water moving into these empty spaces makes the pot vibrate.
|