 Cosmic rays are fast-moving particles that enter the earth's atmosphere in large numbers every day. For example, every person is hit by about half a million cosmic rays every hour. It is common for a cosmic ray to collide with an atom in the atmosphere, creating an energetic neutron; these neutrons collide with nitrogen atoms in the atmosphere. When this happens, a nitrogen-14 atom turns into a carbon-14 atom and a hydrogen atom. Carbon-14 is radioactive, with a half-life of about 5,700 years. (This means that half of it disapears, by turning into something else, every 5,700 years. The physics of radioactive decay is well understood, which is why it can be used for medical imaging, for targeted cancer treatments, for heating spacecraft, as well as for Carbon-14 dating. The ratio of normal carbon (carbon-12) to carbon-14 in the air and in all living things at any given time is nearly constant. As living things excrete it, it gets taken in again from the air. But one the living organism dies, it stops taking in new carbon. This means that the carbon in its remains slowly loses carbon-14 as it decays. The rate of decay is known, so examining old once living artifacts to find out the ratio of carbon-12 to carbon-14 in the sample and comparing it to the ratio in a living organism, it's possible to determine the age of a formerly living thing fairly precisely. 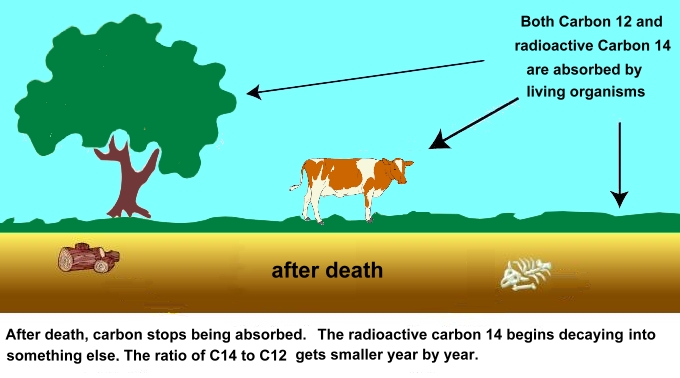 A formula from Math 30 to calculate how old a sample is by carbon-14 dating is: t = [ ln (Nf/No) / (-0.693) ] x t1/2 where ln is the natural logarithm, Nf/No is the percent of carbon-14 in the sample compared to the amount in living tissue, and t1/2 is the half-life of carbon-14 (5,700 years). If you had a fossil that had 10 percent carbon-14 compared to a living sample, then that fossil would be: t = [ ln (0.10) / (-0.693) ] x 5,700 years t = [ (-2.303) / (-0.693) ] x 5,700 years t = [ 3.323 ] x 5,700 years t = 18,940 years old Because the half-life of carbon-14 is 5,700 years, it is only reliable for dating objects up to about 60,000 years old. However, the principle of carbon-14 dating applies to other isotopes as well. Potassium-40, for example, is another radioactive element naturally found in your body and has a half-life of 1.3 billion years. The use of various radioisotopes allows the dating of biological and geological samples with a high degree of accuracy. However, anything that died since the 1950s, when nuclear bombs and open-air nuclear tests started putting radioactive isotopes into the air and ground, will be harder to date precisely. |