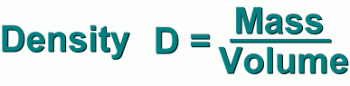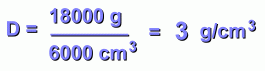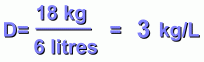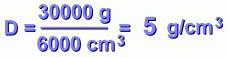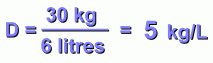On the previous page, you discovered that density measures how much material is packed into a certain volume.
Density can be calculated by measuring a substance's mass, and dividing it by its volume.
Mass can be measured in grams or kilograms. Volume is measured in cubic centimetres or litres.
So density is measured in g/cm3 or kg/L


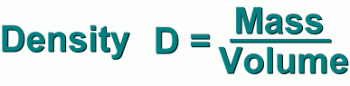
Let's work out the density of a sample substance . . .
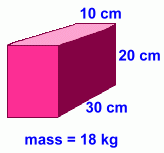 Our sample has dimensions 10 x 20 x 30 cm. Our sample has dimensions 10 x 20 x 30 cm.
This gives it a volume of 6000 cm3, or 6 litres.
Its mass is 18 kg, or 18,000 grams.
First, let's divide grams by cubic centimetres:
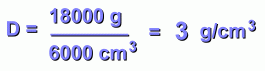
We can also divide kilograms by litres:
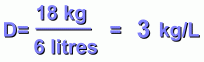
|
Now let's work out the density of another substance of the same size. . .
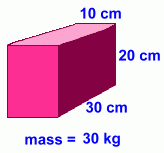 Our sample still has dimensions 10 x 20 x 30 cm. Our sample still has dimensions 10 x 20 x 30 cm.
This makes the volume still 6000 cm3, or 6 litres.
Its mass now is 30 kg, or 30,000 grams.
First, let's divide grams by cubic centimetres:
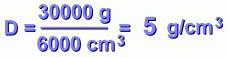
We can also divide kilograms by litres:
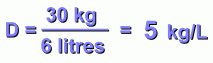
|
  The substance on the left is not very dense. Its density as calculated above was 3 g/cm3.
The substance on the left is not very dense. Its density as calculated above was 3 g/cm3.
The substance on the right is denser. It weighs more, even though it's the same size. Its density is 5 g/cm3.
Page 1 | Main Page
|