
We're going to show you how you can actually measure the thickness, or diameter, of a single molecule. To do this experiment, you'll need a large flat-sided shallow pan of some sort, a straight wire or thin rod at least as wide as the pan, and a small amount of oil of any kind.
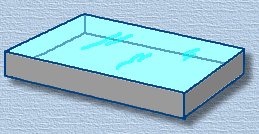 Fill the pan full to the top with water, and place it on a perfectly horizontal surface. Add tiny amounts of water until the water surface bulges slightly over the top of the pan. (This is due to the surface tension forces of the water molecules; this bulging surface is called the meniscus). Fill the pan full to the top with water, and place it on a perfectly horizontal surface. Add tiny amounts of water until the water surface bulges slightly over the top of the pan. (This is due to the surface tension forces of the water molecules; this bulging surface is called the meniscus).
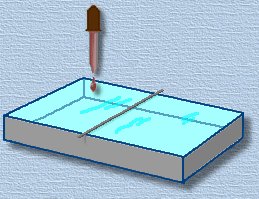 Now carefully lay the straight wire or rod across the pan, as shown. it must touch the water. Add a very tiny drop of oil to the water surface on one side of the wire. We've shown an eyedropper here, but you only want the tiniest amount of oil ... about one cubic millimetre. A tiny drop on the tip of a toothpick will work. Now carefully lay the straight wire or rod across the pan, as shown. it must touch the water. Add a very tiny drop of oil to the water surface on one side of the wire. We've shown an eyedropper here, but you only want the tiniest amount of oil ... about one cubic millimetre. A tiny drop on the tip of a toothpick will work.
You will see the oil spread out across the surface of the water on that end of the pan, right up to the wire. Wait until that end of the pan is completely covered by the oil before proceeding.
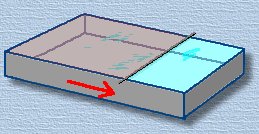 Now slowly move the wire along the length of the pan. As you do, the oil will spread out to cover the larger area. As you move the wire farther, the oil layer will get thinner and thinner. The oil forms a 'blanket' on the surface of the water, and will remain a solid sheet as long as it can, eventually until the blanket is just one molecule deep! You are looking for the point when the oil layer begins to break up; when it does, stop moving the wire. There aren't enough molecules of oil to maintain the blanket. Now slowly move the wire along the length of the pan. As you do, the oil will spread out to cover the larger area. As you move the wire farther, the oil layer will get thinner and thinner. The oil forms a 'blanket' on the surface of the water, and will remain a solid sheet as long as it can, eventually until the blanket is just one molecule deep! You are looking for the point when the oil layer begins to break up; when it does, stop moving the wire. There aren't enough molecules of oil to maintain the blanket.
You now have a layer of oil that is one molecule thick!
(If the oil layer doesn't seem to break up at all, then either start over with an even tinier drop of oil, or find a larger pan. The pan from the bottom of a travel kennel for a large dog might work.)
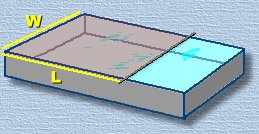 Now for the calculation. Assuming the volume of oil you added was about one cubic millimetre, this volume is now filling a box with dimensions L x W x H, where H is the height of the oil blanket, or the diameter of one molecule. Now for the calculation. Assuming the volume of oil you added was about one cubic millimetre, this volume is now filling a box with dimensions L x W x H, where H is the height of the oil blanket, or the diameter of one molecule.
If the length and width of the oil blanket (the pan) turn are, for example, 400mm and 300mm, the following calculation shows how to solve for H:
V = L x W x H
1 = 400 x 300 x H
0.00000833 = H
|
This makes the height of the oil blanket, or the diameter of one molecule, equal to 0.00000833 mm,
or 0.00000000833 metres. Given the inaccuracy of our measurement, it's probably safe to round this number to 0.00000001 metres, which is one ten-millionth of a metre, or 10-7 m. Since an oil molecule has many atoms in it, this isn't the size of an atom, but the thickness of the entire molecule.
With this simple calculation, we've measured the thickness of one molecule of oil, an object which is too tiny to see in an ordinary microscope!
Resources
|
