|
In the early years of the twentieth century, an unsolved mystery in physics centered around the nature of light. Was light composed of particles, or was it made from waves? Previous experiments by Maxwell had led scientists to believe that light was really a wave. It could produce diffraction patterns; this sort of 'interference' is a property of waves, and not something that could be observed if light was made from tiny particles. Or so it seemed. But there was an experimental result that still remained unexplained. Certain metals would give off electrons when hit by light. This was called the 'Photoelectric Effect'; it could be observed by setting up a circuit which would allow a flow of current only if electrons were moving between two electrodes inside a tube. 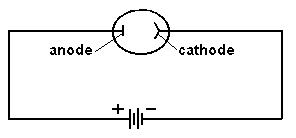 The circuit above will not be complete unless electrons flow from the cathode to the anode. Anything that knocks electrons off the cathode will cause a current to flow in the circuit, ... which can be measured. If light were a wave, it could knock electrons out of a metal without any difficulty. More intense light should knock out more electrons; weak light should knock out few. The wave theory of light predicted that the higher the intensity of the light, the more electrons that would be knocked out of the metal. 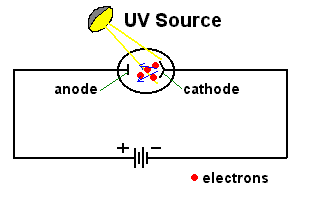 Unfortunately, that wasn't what was observed. If the light hitting the metal was of sufficiently low frequency, no electrons got knocked out at all, even at high intensity! But if the light was high frequency, like ultraviolet, even a very dim beam would easily knock out electrons. Clearly the ability to knock electrons out of a metal depended on the frequency of the light, not its intensity. The theory of light as a wave could not explain this! In 1905, Albert Einstein published a revolutionary theory that explained the photoelectric effect. His idea was that light was actually made from little bundles of energy, [later] called 'photons'. The energy of each little bundle depended upon its frequency; the higher the frequency of the light, the more energy each little bundle carried. These little bundles were wave-like in nature, but also acted like particles! The energy of a photon could be calculated using the equation: By giving light this particle-like behaviour, the photoelectric effect could now be explained easily. An electron sitting in a metal couldn't be knocked out unless the energy it was hit with was big enough. It needed a certain amount of energy to escape, and low frequency light couldn't provide it. Low frequency light didn't have enough energy in its 'bundles', so it couldn't dislodge electrons from the metal. High frequency light, on the other hand, had very energetic 'bundles', which contained enough energy to pass on to the electrons so they could escape from the metal. Want an analogy? O.K., imagine the electron trapped in the metal is like a ball in the bottom of a hole, like this: 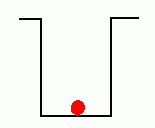 The electron can be given energy if it's hit by a photon of light. But it can only absorb one photon at a time. Photons come with different amounts of energy; let's say, from one to four units:  The electron can be hit by a low energy photon, but the energy it gets isn't enough to let it escape from the hole: 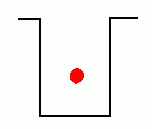 Since the electron can only absorb one photon at a time, and it can't keep the energy if it doesn't leave the hole, no matter how many low-energy photons you hit it with, it can't escape the hole. Similarly, a two-unit photon still won't supply enough energy: 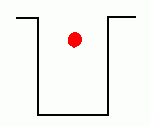 But if the electron is hit by a photon containing three or four units of energy, it gets enough energy to escape from the hole! 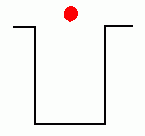 Any left-over energy the electron has after escaping the hole becomes kinetic energy ... the electron moves away with this energy. This can be calculated in the following manner: If fo is the frequency just necessary to knock out an electron, and f is the frequency of the light hitting it, then: Einstein received the Nobel Prize for his explanation of the Photoelectric Effect. |