  There are certain compounds containing silver that react to light by turning dark. This allows us to capture black & white images on film containing these chemicals. This page will show you how to make a simple version of this process, one that's been around for over 150 years. It will illustrate the science of photochemistry that still exists, despite the advent of digital photography.
There are certain compounds containing silver that react to light by turning dark. This allows us to capture black & white images on film containing these chemicals. This page will show you how to make a simple version of this process, one that's been around for over 150 years. It will illustrate the science of photochemistry that still exists, despite the advent of digital photography.You'll need the following ingredients: - bleach - silver polish - some silver that's well tarnished - a cotton cloth - a coffee filter - two small glasses - a coin - a small dim light to work by, in the dark The first step is to dampen the cloth, and then use a small amount of silver polish to thoroughly clean the tarnish off your silver. When you're done, the cloth should be black. Silver tarnish is a chemical called silver sulfide, which forms when pollutants in the air containing sulphur react with silver. You've now transferred this silver sulfide to the cloth. 
 
Now you'll have to work in the dark, with just your small lamp in the background for illumination. Immerse the part of the cloth containing the silver sulfide in a small glass filled with bleach. Stir the cloth in the bleach for a minute or two, so that the silver sulfide goes into the bleach. 

 When you remove the cloth, the bleach should now appear cloudy. What has happened is that the chlorine in the bleach has reacted with the silver sulfide, producing silver chloride. This new chemical is called a photochemical ... it reacts to light by turning dark. The next step is to remove the silver chloride from the liquid by filtering it through a coffee filter into a second glass. Once all the liquid has filtered through, carefully remove the filter paper and open it up. It now contains a thin layer of silver chloride, and will be your undeveloped film. We're going to make a primitive image of the coin, so place the coin on the centre of the filter paper. The final step is to put the wet paper containing the coin outside in direct sunlight for about half an hour. This will 'develop' your silver chloride film, causing that chemical on the surface of the paper to turn dark. The chemical hidden by the coin won't be exposed to the light, so it will stay white. 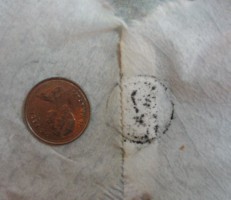 We were surprised when we did this experiment and removed the coin ... the raised part of the metal coin in contact with the silver chloride (or perhaps something on the coin) reacted with the chemical on our 'film' to produce a black outline, nicely highlighting the light outline of the coin. In any case, the part of the film covered by the coin was whiter than the rest of the paper ... a simple photograph of the coin's outline.
We were surprised when we did this experiment and removed the coin ... the raised part of the metal coin in contact with the silver chloride (or perhaps something on the coin) reacted with the chemical on our 'film' to produce a black outline, nicely highlighting the light outline of the coin. In any case, the part of the film covered by the coin was whiter than the rest of the paper ... a simple photograph of the coin's outline.What's happening is that bright light, where it hits the film, is causing it to turn dark. This means that if you use film this way, all the bright areas in your photo will show up dark on the film, and the region hidden from the light will remain light. This produces a photographic 'negative'. By taking a picture of this negative by passing light through it, the same procedure will reverse the light and dark areas on a second piece of film, producing a black and white photograph.  By carefully refining the size and distribution of the silver chloride, photochemists over the past one hundred years have been able to produce high quality photographic reproductions of real things. If the silver chloride particles are small enough, you can reproduce the light and dark areas of things in great detail, as the various levels of light hitting the film cause it to turn darker by varying amounts.
By carefully refining the size and distribution of the silver chloride, photochemists over the past one hundred years have been able to produce high quality photographic reproductions of real things. If the silver chloride particles are small enough, you can reproduce the light and dark areas of things in great detail, as the various levels of light hitting the film cause it to turn darker by varying amounts.
The experiment we've described above is an example of 'wet plate' photography, which was how photography began over 150 years ago. Refinements have also included sealing the silver chloride inside plastic, so the film is dry, and 'fixing' the chemical afer exposure to light, so it will stop 'developing' after taking a picture. And of course, other chemicals can be used to reproduce colours. We got the idea for this experiment from the book: "See for Yourself: More Than 100 Experiments for Science Fairs and Projects", a wonderful collection of projects by Vicki Cobb, published by Scholastic Reference. |