

 The Shrinking Balloon
The Shrinking Balloon

No intro this time ... just an experiment, for which we want you to provide the explanation!
You'll need a small container, similar to a test tube, that has a narrow enough top to be able to pull a balloon over. A small clear plastic bottle might work. You'll also need a balloon, some steel wool, and a little water.
Stuff the steel wool into the bottom of the container. Add a little water. Blow up the balloon and pull the end over the container; it should look like this.
A simple setup, and the balloon should stay inflated for days and days, provided the seal is tight. Except ...
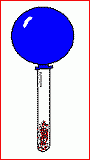 This is what will happen in only a few hours. The balloon has definitely deflated. The seal with the container did not leak. Nothing escaped from the balloon or the container into the air. This is what will happen in only a few hours. The balloon has definitely deflated. The seal with the container did not leak. Nothing escaped from the balloon or the container into the air.
The steel wool seems to have turned a little brown, though ...
What we want you to do is explain why the balloon deflated. Where did the air, or some part of the air, go, if it couldn't escape from the container/balloon enclosure?
|
 Dr. Deadly's Science Lab Question Dr. Deadly's Science Lab Question
"What happened here that made the balloon shrink?
Your answer must explain where the air (or part of it) went."
Scroll way down to check your answer.
Oxygen from the air combined with iron in the steel wool to make
iron oxide. In other words, the oxygen made the metal rust!
|
Science Lab | Resources
|