 Page 2
Page 2
We'll get a little help from a student for this demonstration. Hair is a material that very easily gives up its electrons. Plastic balloons, on the other hand, like to get more electrons. So when you bring the surface of the plastic balloon in contact with your hair (rubbing helps bring more of the surface area into contact), you set up a situation where the electrons in your hair will want to flow onto the balloon.
So what happens when you take the balloon away?
|

|
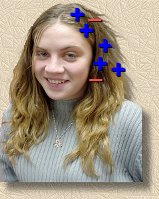
Let's look at her hair first. Because some electrons moved from her hair onto the balloon, there are fewer electrons than there were before, at least on that side of her head.
|
This means that the positive hair nuclei, that were there all along, now outnumber the remaining electrons. So her hair is positively charged, overall.
What does this mean for her hair? Well, the individual hairs are all positively charged. Charges that are the same will move away from each other, or 'repel' each other, if they can. And they can! Individual hairs are light, and free to move. So her hair will tend to look frizzy, or 'staticky', as all the positively charged hairs try to get away from each other. But we're polite, so we won't say anything.
|
What about the balloon? It has a bunch of extra electrons. What effect will that have?
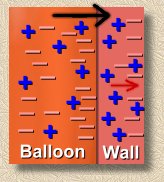
The first picture at the right represents an ordinary neutrally charged balloon that hasn't been anywhere near our volunteer's hair. The negative charges balance the positive charges, so the balloon is overall neutrally charged. Notice that 'neutral' doesn't mean 'no charge' ... it means 'no net charge'. In other words, it's full of both kinds of charges, but the positive and negative effects balance.
Here's the balloon that she rubbed on her hair. it has some extra electrons now. Overall, the negative charges outbalance the positives, so the balloon has a net negative charge. This doesn't do anything noticeable to the balloon ... but it can be the cause of some interesting phenomena!
|


|
If the balloon with all the extra negative charges on it is brought closer and closer to a wall, this extra negative charge causes many of the electrons in the wall to move away from the balloon, deeper into the wall. Remember, 'like' charges repel each other.
The result is that the negatively charged balloon is now close to the surface of the wall, which is positive, because the electrons were pushed away!
|
|
The interesting result? The negatively charged balloon now sticks to the positively charged outer wall, because charges that are opposite attract each other.
Note that the wall was originally neutral. It didn't get any more electrons. The balloon forced some of the wall's electrons to move, leaving behind a positive charge. The balloon was responsible for making the wall 'attractive'. This type of 'charging' is called induction.
This is similar to the reason why a magnet can be attracted to a non-magnetic piece of metal.
|

|
Sometimes when you bring a charged object near to something small and light that can move, (unlike the wall), the smaller object may actually jump towards the bigger one. This is what makes dust, lint, and hairs stick to your pants, which are charged.
 What else can happen when you bring a charged object close to something else? What else can happen when you bring a charged object close to something else?
Well, the 'force' that propels electrons to move can be quite strong. In everyday circumstances, involving normal 'static electricity', this voltage can range anywhere from 1000 to 10,000 volts! This 'force' is so strong that it will propel the electrons over distances of a few centimetres of air. When these electrons pass through the air, they electrify it, causing a noise and a visible spark. That's the 'shock' you get when you're zapped by 'static electricity'.
Fortunately, the electrons involved aren't moving very fast, so there isn't much current flowing when this happens. So you don't get burned.
page 1 | Resources
|