Water Molecules
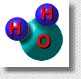 A water molecule is a chemical bonding of two hydrogen atoms with an oxygen atom. Its formula is H2O. A water molecule is a chemical bonding of two hydrogen atoms with an oxygen atom. Its formula is H2O.
Atoms can have electrical charges, but overall the total number of negative and positive charges on a water molecule are equal, so the molecule is neutral.
|
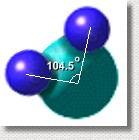
But it's not as simple as that. A water molecule is not symmetric ... the two hydrogen atoms are stuck on one side of the oxygen atom, separated by an angle of 104.5°. The negative charges are mostly on the oxygen atom, leaving the hydrogen atoms mostly positive.
The result is that one side of the water molecule is negative, and the other is positive.

Molecules like this are called polar molecules. This polarity will have some interesting consequences with respect to the properties of water.
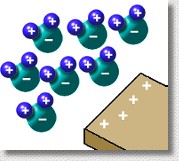 Polar molecules in a liquid make that liquid attractive to electrostatically charged objects. When a charged object (in this case, a positively charged piece of plastic) is brought near to some water, the molecules orient themselves so that the negative sides of the molecules are facing the large positive charge. This creates an attractive force between the water and the plastic. Polar molecules in a liquid make that liquid attractive to electrostatically charged objects. When a charged object (in this case, a positively charged piece of plastic) is brought near to some water, the molecules orient themselves so that the negative sides of the molecules are facing the large positive charge. This creates an attractive force between the water and the plastic.
You can observe this yourself by charging a comb (run it through someone's dry hair) and holding it near to a stream of water from a tap. The stream of water will bend towards the comb.
|
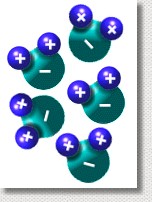 The polarity of water molecules also helps them to stick close together. The slightly negative oxygen atoms in all the molecules are attracted to the slightly positive hydrogen atoms in all the nearby molecules. This attractive force between molecules keeps them relatively close together; the force is called hydrogen bonding. The polarity of water molecules also helps them to stick close together. The slightly negative oxygen atoms in all the molecules are attracted to the slightly positive hydrogen atoms in all the nearby molecules. This attractive force between molecules keeps them relatively close together; the force is called hydrogen bonding.
Hydrogen bonds are small forces ... much smaller than the force which holds the molecule together. But they are much stronger than the forces between molecules in other substances that don't have hydrogen bonds.
This gives liquid water some properties not shared by other liquids. Because of the attractive force between molecules, water molecules would much rather stick to each other than something else. Water puddles, doesn't wet things easily, and has high surface tension.
 You can see another property yourself with a simple experiment. Fill a glass with water to the very top. Then slowly add tiny objects one by one (a few pennies), and examine the rim of the glass. You will see the water bulging up over the walls of the container. This bulge is called the meniscus, and is also visible at any water level if you look very closely. It can make reading the volume level of water in a graduated cylinder a liitle inaccurate ... do you read the level at the top of the bulge, or at the bottom? (Actually, you're supposed to take a reading half way between). You can see another property yourself with a simple experiment. Fill a glass with water to the very top. Then slowly add tiny objects one by one (a few pennies), and examine the rim of the glass. You will see the water bulging up over the walls of the container. This bulge is called the meniscus, and is also visible at any water level if you look very closely. It can make reading the volume level of water in a graduated cylinder a liitle inaccurate ... do you read the level at the top of the bulge, or at the bottom? (Actually, you're supposed to take a reading half way between).
The bulge forms because the water molecules are more attracted to each other than the walls of the glass. At the edges, they pull away towards each other, rather than the glass.
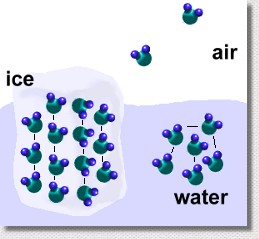 The hydrogen bonds between molecules of water in all its forms also explain some other properties of water we have already seen. In solid form, the ice molecules line up, and the hydrogen bonds are at maximum strength. In order to melt the ice, much energy is needed to bend and break these bonds ... this explains ice's high melting point, and water's high heat of fusion.
In liquid form, the molecules are still attracted to one another, but less strongly, and the bonds don't form straight lines. Hydrogen bonds with nearby molecules are continually being formed, broken, and reformed, as the molecules slide past each other. In order to evaporate the water, much energy must be supplied to break all of these bonds. This explains water's high boiling point, and water's high heat of vaporization.
The hydrogen bonds between molecules of water in all its forms also explain some other properties of water we have already seen. In solid form, the ice molecules line up, and the hydrogen bonds are at maximum strength. In order to melt the ice, much energy is needed to bend and break these bonds ... this explains ice's high melting point, and water's high heat of fusion.
In liquid form, the molecules are still attracted to one another, but less strongly, and the bonds don't form straight lines. Hydrogen bonds with nearby molecules are continually being formed, broken, and reformed, as the molecules slide past each other. In order to evaporate the water, much energy must be supplied to break all of these bonds. This explains water's high boiling point, and water's high heat of vaporization.
Water
Resources
|