  Aerogels are produced by removing the liquid component of a gel by freeze-drying. This allows the liquid to be slowly dried off without causing the solid matrix of the gel to collapse. Despite the name, aerogels are solid, rigid, and dry materials that do not resemble a gel in their physical properties.
Aerogels are produced by removing the liquid component of a gel by freeze-drying. This allows the liquid to be slowly dried off without causing the solid matrix of the gel to collapse. Despite the name, aerogels are solid, rigid, and dry materials that do not resemble a gel in their physical properties. Pressing softly on an aerogel doesn't leave a mark, but if you press harder you will leave a permanent depression. Press too hard, though, and the substance might shatter like glass. (This property is known as friability)  Despite this, aerogels are very strong structurally. They are also good thermal insulators because they nullify two of the three methods of heat transfer. They are good insulators against conduction because they are composed almost entirely of gases, which are very poor heat conductors. They are insulators against convection because air cannot circulate through the structure of an aerogel. However, aerogels are poor insulators against radiation (heat) because infrared radiation passes right through them.
Despite this, aerogels are very strong structurally. They are also good thermal insulators because they nullify two of the three methods of heat transfer. They are good insulators against conduction because they are composed almost entirely of gases, which are very poor heat conductors. They are insulators against convection because air cannot circulate through the structure of an aerogel. However, aerogels are poor insulators against radiation (heat) because infrared radiation passes right through them. Aerogels are hygroscopic (moisture absorbing); they feel dry and are a strong desiccant. People handling aerogel for extended periods should wear gloves to prevent the appearance of dry brittle spots on their skin. I ordered a small sample of aerogel because I was curious about its properties:   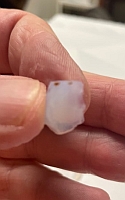 Despite it's crystalline appearance, it doesn't feel like crystals at all because the pieces are so light. They are cloudy, and definitely solid. They are also most definitely a strong dessicant; after handling a few pieces for just sixty seconds my fingertips became very dry, so much so that they felt uncomfortable. 
The index of refraction for silica aerogel is supposed to be 1.08, which is very low, close to that of air. Without the necessary equipment to actually measure that, I was, however, still able to confirm by eye using a laser pointer that the refraction is so slight as to be unnoticeable. There are many applications for aerogels. Here are a few:

|