 A solution is a mixture that is very thoroughly mixed! This happens when you mix two liquids, or dissolve a solid in a liquid. In the latter case, the solid breaks up completely ... into molecules! 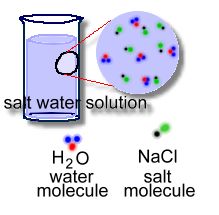 Dissolving a solid ( a solute) into a liquid solvent (eg: water) to make a solution happens when the attraction between the particles of the solvent and solute are strong enough to overcome the attraction of the particles of the solid for one another. This lets the solvent pull apart the solid into molecules, which can then completely intermingle, molecule by molecule, with the solvent molecules! You could never stir a cake mix enough to make it this 'smooth'; there are no lumps at all. Only molecules. The components of a solution (the solute and the solvent), cannot be separated by filtration or by any physical methods. This is because both solute particles and the solvent molecules are very small as compared to the pores in the filter paper. However, a solution can be separated using distillation, or evaporation. When distilling, you heat up the solution so that the component with the lowest boiling point evaporates first, leaving the other solutes behind. This could be as simple as boiling a solution of salt water, where the water evaporates, leaving the salt behind as a residue. This method also works to separate solutions of two or more liquids from each other. This is how, for example, crude oil is separated into various liquids and gases, such as heavy oil, naptha, kerosene, and gasoline. Separation involves piping crude oil through hot furnaces; inside distillation units the liquids and vapours separate into petroleum components, called fractions, according to their boiling points. Heavy fractions are on the bottom and light fractions are on the top. Learn more about this process by watching an excellent video here. their component molecules and completely intermixed.' |