  A molecule made from three different atoms. There are two main types of chemical bonds in chemistry: covalent bonds and ionic bonds. Chemical bonds hold atoms together in a molecule, and occur when two or more atoms either share or donate electrons. Each molecule in a compound can have one or more types of atoms in it. Because the atoms are actually joined together, you cannot separate the substances in a compound by hand, by filtering, or by any mechanical means. Some examples of some simple compounds, with their corresponding formulas:
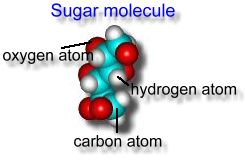 Each molecule is made up of 3 kinds of atoms stuck together in a certain pattern. The substance 'sugar' is just a collection of many of these molecules:  'A compound is made from molecules, which are chemical combinations of atoms of one or more types. A compound can't be separated into different substances by mechanical means' |