 The word 'laser' is actually an acronym which stands for Light Amplification by Stimulated Emission of Radiation. Laser light is produced by a source that emits light photons in a coherent beam, with all the photons having the same wavelength. Regular light, by comparison, is usually emitted in all directions, and with a wide variety of wavelengths. Laser light is not limited to photons in the visible spectrum; it can be in the form of x-rays, infrared, ultraviolet, microwave, radio, and other wavelengths. Lasers are used in a wide range of products and technologies. You will find them in CD players, dental drills, cutting machines and measuring systems. The first common application of lasers was the supermarket barcode scanner, introduced in 1974. The compact disc player was the first laser-using device to become common in the home, beginning around 1982. In order to understand how lasers work you have to know something about atoms. There are about 100 different kinds of atoms, each with its own nucleus made from protons and neutrons. In this simple view of an atom, each one has electrons orbiting its nucleus. These electrons follow paths at different levels, based on how much energy they have.  Energy can be added to an atom from outside, by applying heat, electricity, or radiation; this will cause the atom's electrons to gain energy, or increase their energy levels. Electrons which have had energy added are called 'excited'. Energy can be added to an atom from outside, by applying heat, electricity, or radiation; this will cause the atom's electrons to gain energy, or increase their energy levels. Electrons which have had energy added are called 'excited'.
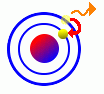 Similarly, electrons can lose some of their energy and drop to a lower level. When they do, the atom will emit this excess energy in the form of photons (electromagnetic energy). Similarly, electrons can lose some of their energy and drop to a lower level. When they do, the atom will emit this excess energy in the form of photons (electromagnetic energy).
Although this simplified picture of an atom is not actually the true picture of what happens, it is a useful one to help describe how lasers work. Let's summarize: - When energy hits an atom, it causes its electrons to jump to higher orbital levels. - When an electron falls to a lower level, it gives off excess energy in the form of a photon of light You can actually see the effects of atoms releasing light as their electrons fall to lower orbital levels. When the heating element on a stove turns bright red, the light you see is caused by atoms that were excited by heat and are now giving off red photons. When you see a picture on an old-style television screen or monitor, what you are seeing is atoms, excited by high-speed electrons, emitting many colours of light. In fact, just about anything that produces light does it when electrons drop to lower orbits and release photons. A laser is a device that controls the way these energized atoms release their photons. A laser contains some sort of medium, either a gas, liquid or solid, with a source of atoms that will be pumped full of energy. Intense bursts of electricity or light cause the electrons in these atoms to become excited and jump to identical higher levels. 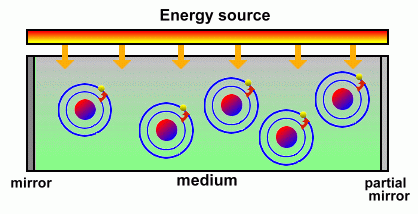 Once the medium contains a large number of atoms with electrons excited to the same levels, it is ready to produce laser light. Some electrons will begin to fall to a lower level, releasing photons of light as they do. These photons will all share the same wavelength and will all vibrate in the same direction. Specifically, they share the same wavelength, phase, and polarization. 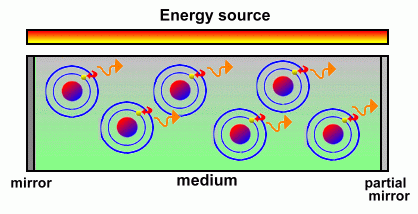 When a released photon hits another excited atom that is at the same energy level, it causes that atom's electrons to drop as well and release an identical photon. This is called 'stimulated emission'. Pretty soon you have huge numbers of photons that have been released, and all share the same wavelength and vibration direction because they have all been released by stimulated emission. (In a regular flashlight, by comparison, all of the atoms release their photons randomly). Both ends of the medium containing the atoms and their excited electrons are mirrored. As more and more electrons lose their energy and fall to lower levels, an increasing number of emitted photons starts to fill the region. The mirrors keep this light inside, bouncing from one end to the other, until a sufficient quantity exist.  One mirror is actually only partially reflective; when the number of photons reaches a certain point the light can escape through that mirror, producing a sudden flash of light energy. All of the photons coming out have the same wavelength and all are vibrating in the same direction. This 'coherent' light beam is laser light. One mirror is actually only partially reflective; when the number of photons reaches a certain point the light can escape through that mirror, producing a sudden flash of light energy. All of the photons coming out have the same wavelength and all are vibrating in the same direction. This 'coherent' light beam is laser light.
By continually supplying energy to the atoms in the medium (pumping it), the energy levels can be continually raised back to a higher level, allowing repeated buildups of released photons and a continuous beam of these photons as they escape from the medium. The quantum mechanical effect called 'stimulated emission', where a photon of a certain wavelength emitted by an atom will stimulate atoms in an identical state to release identical photons, was discovered by Albert Einstein while researching the photoelectric effect. This is how he earned his Nobel Prize in Physics. A laser beam will spread much less than a beam of ordinary light. It's analogous to a group of soldiers marching in lock step in the same direction, compared to a random group of people wandering together and jostling each other. A beam of laser light generated by a small lab laser spreads to only about 1.6 kilometres in diameter if shone from the Earth to the surface of the Moon! [We wondered about the spread from a simple laser pointer, if it were strong enough to illuminate the surface of the moon and be detected. If you're interested, check out our calculations here.] Here's another analogy: imagine a group of hundreds of people exiting a doorway in a building. Under ordinary circumstances the people, each moving their legs by different amounts and in different directions, will spread out into a huge mass as they move away from the building. This corresponds to the behaviour of ordinary light containing waves of many different frequencies. Now imagine that the people leaving the building are soldiers marching in ranks and in step. Their leg movements and direction are identical; the soldiers will leave the building in ranks and will hardly spread apart at all. This corresponds to a beam of laser light where the waves all have the same frequency, phase and polarization. In summary, here are the characteristics of laser light:
 Some lasers are very powerful; a CO2 laser can cut through steel. These lasers are dangerous because they emit light in the infrared and microwave region of the spectrum. This laser light can melt its way through whatever it is focused on. All lasers, even low-power laser pointers, are dangerous if the beam directly enters your eye. Laser light, like all light, is invisible if it doesn't enter our eye. We can only see light that has reflected off something. Beams of light in air can't be seen, but can be made visible if they pass through dust or water vapour. In the pictures below, an invisible laser beam is shown in air, and then passing through a mist of water droplets. Reflections off the water droplets are able to bounce back and enter your eye (or in this case the camera lens) and be seen. 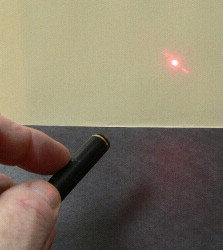  |