 Methane is a colourless, odourless gas that is found in many places in the environment. It is the main component of natural gas, a mixture containing about 75% methane (CH4), as well as 15% ethane (C2H6), and 5% other hydrocarbons. Methane is produced on Earth mainly through the anaerobic ('without oxygen') decomposition of plant and animal matter by bacteria. This decomposer is at work everywhere, helping dead organic matter to rot, and producing methane on the ground, and especially in landfills. Natural gas, which is about 75% methane, is used as an energy source. Your home may be heated by natural gas. A trace amount of a smelly sulfur compound (tertiary-butyl mercaptan and dimethyl sulfide) is added to natural gas, to give it a detectable odour in case of leaks. Natural gas and methane are not poisonous if breathed, but they displace oxygen from the air, and will cause suffocation, as well as being highly explosive. Methane can be manufactured commercially by heating bituminous coal (see below). In the chemical industry, methane is a raw material used in the manufacture of methanol, formaldehyde, chloroform, carbon tetrachloride, and other chemicals. But the principal use of methane is as a fuel, for heating and to produce electric power. Here are some diagrams showing the chemical structure of the methane molecule. It is made up of a single carbon atom, joined by covalent bonds to four hydrogen atoms. Carbon has a shortage of four electrons in its outer shell; each hydrogen atom provides an electron to share with each of these 'vacancies'. 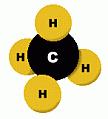 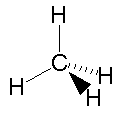 Methane is the primary constituent of natural gas, a common heating fuel. Natural gas can be found in deposits beneath the surface of the earth, often in conjunction with petroleum deposits. Before it is distributed, natural gas is processed. The heavier hydrocarbons (propane and butane) are removed first. Contaminants like hydrogen sulfide (which smells bad, is highly poisonous, and is the dangerous component in 'sour gas'), must also be removed. The clean gas is then distributed by pipeline. Methane can also be produced by heating coal, a combustible rock formed from the remains of decayed vegetation. Coal that contains more than 15% burnable material is called bituminous coal. When coal is burned in a controlled fashion, various substances can be collected, including methane, water, carbon dioxide, ammonia, benzene, toluene, naphthalene, oils, tars, and sulfur-containing products. What remains is mostly carbon, called 'coke', and is itself an excellent fuel. However, it may contain highly toxic metals such as arsenic and lead. Obviously, uncontrolled burning of coal as a fuel will release all of these pollutants into the atmosphere, unless they are collected by various filtering methods. Animal burps and farts contribute an astonishing 75 million tons of methane gas into the atmosphere each year. Much of the world's livestock are ruminants, such as sheep, goats, camel, cattle, and buffalo. They have a unique, four-chambered stomach, one of which is called the rumen. In an animal's rumen, bacteria break down food ... and release methane as a by-product. A newly discovered source of methane is in the form of methane hydrates. These are chemical compounds containing methane, which are found in arctic ice and seafloor sediments. Some researchers estimate there may be enough energy from this source to supply us for hundreds, maybe thousands, of years. However, little is known at present about their location or quantity. |