
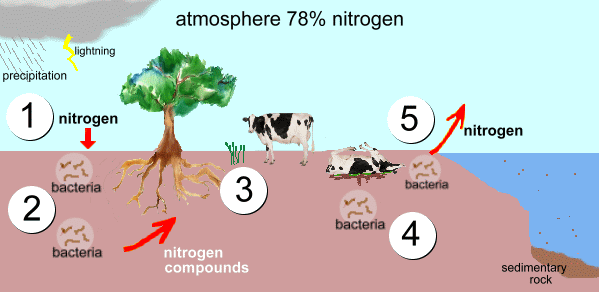
The nitrogen cycle is a combination of biological, geological and chemical processes through which nitrogen is converted into many forms, passing from the atmosphere to the soil to organisms and back into the atmosphere again.
Organic nitrogen exists in living organisms, and gets passed through the food chains by the consumption of other living organisms.
Inorganic nitrogen is found in abundance in the atmosphere. This nitrogen is made available to plants by bacteria in the soil which can convert nitrogen into a usable form.
An overview of the Nitrogen Cycle
Nitrogen is abundant in the atmosphere, but it is unusable to plants or animals unless it is converted into nitrogen compounds. Nitrogen-fixing bacteria play a crucial role in changing atmospheric nitrogen into nitrogen compounds that can be used by plants.
The plants absorb the usable nitrogen compounds from the soil through their roots. Then, these nitrogen compounds are used for the production of proteins and other compounds in the plant cells.
Animals obtain nitrogen by consuming these plants, or other animals.
During the final stages of the nitrogen cycle, after the animals die, bacteria and fungi help decompose the organic matter, and the nitrogen compounds get dissolved into the soil where they are again available for use by plants.
Some bacteria also convert some of these nitrogen compounds in the soil and turn them into nitrogen gas. Eventually, this gets back into the atmosphere.
These sets of processes repeat continuously, and so maintain the percentage of nitrogen in the atmosphere at 78%.
|
The Nitrogen Cycle consists of the following steps:
1. Nitrogen fixation 2. Nitrification 3. Assimilation 4. Ammonification 5. Denitrification.
- Nitrogen Fixation
This is the initial step of the nitrogen cycle. Atmospheric nitrogen N2, which is mostly inert, is deposited into the soil from the atmosphere, mainly through precipitation. There, bacteria combine gaseous nitrogen with hydrogen to form ammonia (NH3).
Other methods of Nitrogen Fixation include:
- atmospheric fixation, where the energy of lightning breaks nitrogen into nitrogen oxides, which are then used by plants.
- Industrial nitrogen fixation, where ammonia is manufactured by combining nitrogen and hydrogen. Later, it is converted into various fertilizers, such as urea.
- Nitrification
In this process, the ammonia is converted into nitrates by the presence of other bacteria in the soil. This conversion is very important as ammonia gas is toxic for plants.
- Assimilation
Primary producers (plants) take in the nitrogen compounds from the soil with the help of their roots. These include nitrite ions, nitrate ions and ammonium ions, and all are used in the formation of the plant and animal proteins. Nitrogen enters the food web when the primary consumers eat the plants.
- Ammonification
When plants or animals die, the nitrogen present in the organic matter is released back into the soil. The decomposers, bacteria or fungi present in the soil, decompose the organic matter back into ammonia, which is used for other biological processes.
- Denitrification
Denitrification is the process by which the nitrogen compounds make their way back into the atmosphere by being converted into gaseous nitrogen (N). This process of the nitrogen cycle is the final stage. Denitrification is carried out by more bacteria, which process nitrates to gain oxygen, and give out nitrogen gas as a byproduct.
Nitrogen Cycle in the Marine Ecosystem
The process of the nitrogen cycle occurs in the same manner in the marine ecosystem as in the terrestrial ecosystem. The only difference is that it is carried out by marine bacteria.
The nitrogen-containing compounds fall into the ocean as sediments get compressed over long periods and form sedimentary rock. Due to geological uplift, these sedimentary rocks move onto land. Recent research has shown that the nitrogen from these rocks is released into plants as the rocks weather.
Resources
HTML, graphics & design by Bill Willis 2024
|

