 When you point the end of the box with the slit in it at a source of light, the light beam will enter the box, reflect off the CD, and exit through the viewing tube. The many fine lines on the CD, which act as a diffraction grating, cause the light to disperse, or spread out. (The same effect can be caused by sending light through a prism). Each of the different wavelengths that were in the original beam bend by a different amount. When you see this 'split-apart' beam in the viewing tube, you will see all these different wavelengths that were in the original light.  Continuous Emission Spectrum
Continuous Emission Spectrum
Because sunlight contains all the wavelengths of visible light, light from the sun will split into a continuous spectrum of colours, like a rainbow. [DO NOT aim the spectroscope at the sun; even a reflected image of the sun will damage your eyes]. This is also true for light from ordinary incandescent light bulbs, where the light comes from the glowing metal (tungsten) filament. At the right is a photo taken through the viewing tube of our spectroscope, which was pointed at a bright incandescent light bulb. A continuous spectrum (rainbow) is clearly visible. (We didn't enhance these photos in any way; the image was clear and easy to photograph. Focusing while simultaneously holding the box and pressing the shutter, however, was somewhat tricky). Non-Continuous Emission Spectrum When a glowing gas gives off light, the light comes from the atoms that are in that gas. Every type of atom gives off a unique wavelength of light when its electrons fall into place. Examining the spectrum from a glowing gas will show bright lines at any wavelength where light is being emitted. That's similar to what happens inside a fluorescent light. Some of the light produced is broad-spectrum, so you'll still see the full rainbow. But gas in the fluorescent light glows at a wavelength characteristic to that gas, and will show up as bight bands in the spectrum. Below are two spectra from fluorescent lights. The first one is from common overhead long fluorescent tubes, the kind that are in every school. There is a clear band of light in the green area corresponding to a gas emitting light inside the tube. The second one is from a small fluorescent bulb in a document viewer, which gives off bright white light; more than one band is visible here.  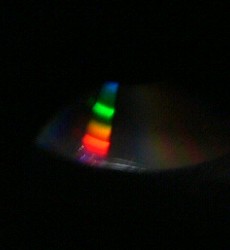 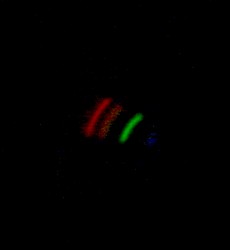
The photo at the right is the spectrum of the light from the LCD display screen of a laptop computer. The screen was set to white, and then the spectroscope was used to spread the wavelengths apart. The spectrum clearly shows that the light produced by the tiny crystals on an LCD screen is made from just three or so different wavelengths. Additional light sources you might want to look at through your spectroscope might include the flame from a butane lighter, or the flame from a laboratory gas jet. In either of these cases, adjust the flame so that it is mostly blue, so you'll get the light from the burning hydrocarbons. Uses for a Spectroscope You can identify an unknown material by burning some of it! By first making spectrum photos of all the single-element gases (like for example, argon or neon), you can use them to compare to the spectrum of an unknown sample. This will let you identify what gases must be glowing in the sample by the bands that are produced. Another interesting and useful phenomenon is that any gas between you and the glowing light will absorb some of the light at wavelengths characteristic to that gas, producing dark bands in the spectrum. As a result, the spectrum of ordinary sunlight isn't really continuous (despite what we told you earlier). As light from the sun passes through cooler upper layers of gas at its surface, some of the light wavelengths are absorbed by these gases. The actual spectrum of the sun, examined in detail, shows many such dark bands, each corresponding to an element in the sun's surface. It was by examining these dark bands (called Fraunhofer lines after their discoverer) that the composition of the upper layers of the sun was first determined. Using both bright bands from emitting atoms and dark bands from absorbing atoms, scientists have also been able to determine the chemical composition of stars and interstellar gases (which are thousands of trillions of kilometres away) by passing star light through a spectroscope. |