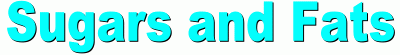
Sugars The simplest carbohydrates are simple sugars, the most common of which is a sugar called glucose. The formula for glucose is C6H12O6. It's a molecule that contains 6 carbon atoms, 12 hydrogen atoms, and 6 oxygen atoms. A glucose molecule looks like this: 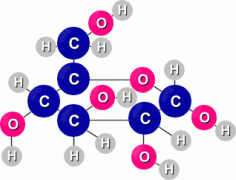 Another simple sugar is called fructose, or fruit sugar. Here are diagrams of both the glucose and fructose molecules, so you can see the difference: 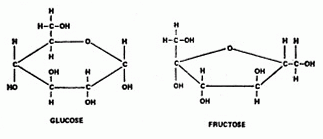 If you count atoms, you'll discover that both simple sugars glucose and fructose contain 6 carbon atoms, 12 hydrogen atoms, and 6 oxygen atoms. They both share the same chemical formula C6H12O6. The difference (which isn't indicated by the formula) is in how the atoms are arranged in each molecule. Complex carbohydrates are molecules made when several simple sugars are joined together by covalent bonds to make a bigger molecule. When two simple sugars join, the molecule is called a disaccharide ('double sugar'). One example is maltose, or malt sugar, which is a molecule made from two glucose molecules. A more familiar example is sucrose, or table sugar, which is made up of one fructose and one glucose molecule. The sucrose (ordinary table sugar) molecule looks like this: 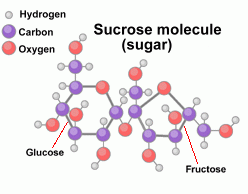 When you're cooking, some recipes may call for a small amount of lemon juice. Often this is added to cause the sucrose in another ingredient to break down into fructose and glucose. Adding honey instead means you can leave out the lemon juice, because honey is already a mixture of separate molecules of fructose and glucose. What makes a sugar sweet? Sweetness is due to the O-H atom combinations which stick out from the sides of the sugar molecule, which react with taste buds on our tongue. And it's not how many of these O-H groups there are that matter to 'sweetness', but where they're located. Only certain orientations of the O-H branches can fit onto the sweet receptors on the tongue. Scientists have learned to manufacture non-sugar substances that contain OH groups arranged similarly on the surfaces of these molecules, which make them 'taste' sweet even though they aren't sugar. We use them as artificial sweeteners.  Other complex carbohydrates can be formed by long chains of simple sugars joined together (polysaccharides). For example, both starch and cellulose are molecules made up of many glucose molecules joined together. These long chain molecules make the substances they are in stiffer. Other complex carbohydrates can be formed by long chains of simple sugars joined together (polysaccharides). For example, both starch and cellulose are molecules made up of many glucose molecules joined together. These long chain molecules make the substances they are in stiffer. The long starch molecule is the basic ingredient in pasta. Cellulose is the molecule that makes up wood fibers. Most mammals (including humans) can digest starch but not cellulose. The difference in the two long molecules is in the way the glucose molecules join together. Digestion is aided in each case by enzymes Plants store the food they make as starch molecules. Animals store their food as molecules of glygogen, another type of chain molecule made from many glucose molecules. Lipids 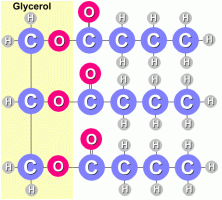 Lipids are long molecules that are also made from just carbon, hydrogen, and oxygen atoms. Lipids make up butter, fats, oils and waxes.
Fats and oils are made from three fatty acid molecules bonded to a glycerol molecule.
Lipids are long molecules that are also made from just carbon, hydrogen, and oxygen atoms. Lipids make up butter, fats, oils and waxes.
Fats and oils are made from three fatty acid molecules bonded to a glycerol molecule.
A fat molecules is shown at the right. Each attached fatty acid molecule is made from a hydrocarbon chain that looks like this: 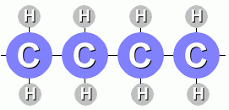 There are actually two kinds of fats. Saturated fats have single bonds between the carbon atoms: 
These molecules are saturated with hydrogen atoms; all the carbon atoms (except on the ends) have two hydrogen atoms attached. Unsaturated fats have a double bond between two carbon atoms: 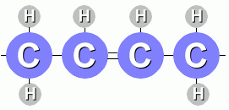
The carbon atoms are not completely saturated with hydrogen atoms. Some fats have double bonds between two or more carbon atoms ... these are called polyunsaturated fats. Animals store energy in saturated fats; plants produce mostly unsaturated fats. Saturated fats lead to a disease in humans called atherosclerosis, because those fatty acids tend to stick to artery walls and interfere with blood flow. Oils containing fats from vegetables don't seem to do this. |