 In a living organism, cells perform many of their functions by carrying out chemical reactions. Chemical reactions happen quickly in a cell; you may know that heat helps reactions occur faster ... but too much heat destroys biological compounds. Cells need another method for making their reactions happen more quickly. In the laboratory, catalysts are used to speed up chemical reactions. A catalyst is a chemical which allows a reaction to occur more quickly, without being involved in the reaction itself. For example, hydrogen and oxygen will react to form water, but this reaction will happen much more quickly, and at a lower temperature, if platinum is present as a catalyst. In living organisms, within a cell, there are special types of molecules that act as catalysts for biological chemical reactions. These molecules are proteins called enzymes. Enzymes allow chemical reactions within cells to occur quickly, and at the relatively low temperature inside cells.   In order for chemical reactions to occur, something must first provide the 'activation energy'. This is the small amount of energy needed to get the reaction going. It's like lighting a campfire. Before the wood will start to burn (the chemical reaction), a small bit of energy from a match is needed to start things off. This match provides the activation energy. In order for chemical reactions to occur, something must first provide the 'activation energy'. This is the small amount of energy needed to get the reaction going. It's like lighting a campfire. Before the wood will start to burn (the chemical reaction), a small bit of energy from a match is needed to start things off. This match provides the activation energy.
Imagine that you wanted to make lighting the campfire easier. You might do this by splitting some of the wood apart and holding the match right next to the bare wood. This would mean you no longer need a big kitchen match ... now a small paper match will provide enough heat to start the wood burning. That's what an enzyme in a cell does. It brings together the chemicals that are going to react and lowers the activation energy necessary to get the reaction underway. It makes it easier for the reaction to occur, and at a lower temperature. 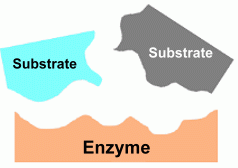
Here's how enzymes do this. First, let's use the proper terms for a cellular reaction. -The substrates are compounds in the cell that are going to react chemically. - The enzyme is the catalyst that will make it easier for the reaction to happen. - The product is the compound that is formed by the reaction. Because the enzyme is just a catalyst, it does not participate in the reaction, and is not used up; it just helps things along. In the example above, an enzyme is helping two substrates to react chemically to form a single product. Our example could just as easily have shown an enzyme taking a single substrate and allowing it to react by splitting into several products. Any sort of reaction is possible and may be helped out by the proper enzyme.  The 'lock and key' theory explains how an enzyme's shape is responsible for helping a reaction between two substrates happen. An enzyme's surface shape is a pattern that the substrates match exactly. This allows the substrates to fit closely together as they attach themselves to the enzyme. Being so close together, the two compounds can now enter into the chemical reaction much more easily. The place on the enzyme where the substrates fit is called the active site.
The 'lock and key' theory explains how an enzyme's shape is responsible for helping a reaction between two substrates happen. An enzyme's surface shape is a pattern that the substrates match exactly. This allows the substrates to fit closely together as they attach themselves to the enzyme. Being so close together, the two compounds can now enter into the chemical reaction much more easily. The place on the enzyme where the substrates fit is called the active site.
The enzyme helps a chemical reaction to happen by bringing the substrates close together. 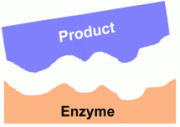 Once the reaction has occurred, the resultant product is now free to leave the enzyme.
Once the reaction has occurred, the resultant product is now free to leave the enzyme.Clearly for enzymes to work this way, an individual enzyme can only control one type of reaction. Conversely, every type of reaction that occurs within a cell may need its own separate enzyme. For example, the enzyme that breaks down starch cannot break down cellulose; it has the wrong shape. A different enzyme would be needed for that. Synthesis vs Hydrolysis: As mentioned above, some enzymes help substrates combine into a single product, while others may help a single substrate break down into several products. Saying this a different way, some enzymes build large molecules from smaller ones, while other enzymes break down large molecules into smaller ones. Synthesis occurs when an enzyme combines several smaller molecules into a large one. Common examples include enzymes that help simple sugars combine to form polysaccharides, amino acids to form proteins, and nucleotides to form nucleic acids. In biological processes, this almost always involves the formation of water molecules from the 'leftover pieces', and so is called dehydration synthesis. Hydrolysis occurs when a large molecule is broken down into smaller ones. Water molecules are used to 'seal the edges' of the new smaller molecules , so synthesis is the opposite of dehydration synthesis. Synthesis is also know as digestion. Factors Affecting How Enzymes Work: Temperature Enzymes will do their job over the range of temperatures prevalent in the cell environment. For living organisms this is body temperature. If it gets too hot, however, an enzyme may no longer be able to help reactions occur. This is because at higher temperatures the protein molecule that is the enzyme may start to 'unravel' as its bonds break, and its surface may no longer be the correct shape for the substrates to fit. pH The pH of the cell environment will determine how effectively the enzyme can do its job. Some enzymes (those in the stomach, for example) require an acidic environment in order to work. Others (for example, in the intestine) require conditions that are basic. Each enzyme in an organism has its own pH requirement. If the pH changes, it may no longer be able to facilitate the chemical reactions within the cell. |