 Thermodynamics is a science that measures and describes the relationship between heat, work, temperature, and energy. It deals with the transfer of energy from one place to another and from one form to another. The key concept is that heat is a form of energy corresponding to a definite amount of mechanical work.  Heat was not recognized as a form of energy until about 1798, when Benjamin Thompson, a British military engineer, noticed that large amounts of heat could be generated in the boring of cannon barrels, and that the amount of heat generated is proportional to the work done in turning a boring tool.
Heat was not recognized as a form of energy until about 1798, when Benjamin Thompson, a British military engineer, noticed that large amounts of heat could be generated in the boring of cannon barrels, and that the amount of heat generated is proportional to the work done in turning a boring tool. His observation of the proportionality between heat generated and work done lies at the foundation of thermodynamics. Another pioneer was the French military engineer Sadi Carnot, whose work concerned the limitations on the maximum amount of work that can be obtained from a steam engine operating with a high-temperature heat transfer. Later that century, these ideas were developed by Rudolf Clausius, a German mathematician and physicist, into the first and second laws of thermodynamics. There are four laws in thermodynamics, perhaps the most important laws in all of physics. Here they are: Zeroth law of thermodynamics If two thermodynamic systems are each in thermal equilibrium with a third, then they are in thermal equilibrium with each other. The Zeroth law is named the way it is because its importance wasn't understood until after the other three laws had been named. This law is so fundamental that it had to go before the others. The law says that if A=B and C=B, then A=C. This may seem so obvious that is doesnít need stating, but this property makes it meaningful to use thermometers as the 'third system', and to define a temperature scale. First law of thermodynamics Energy can neither be created nor destroyed. It can only be changed from one form to another. In any isolated system, the total energy remains the same. The energy in an isolated system can be converted to heat or work or other things, but you always have the same total that you started with. Note that Earth is NOT an isolated system, as it receives energy from the Sun. As an analogy, think of energy as indestructible blocks. If you have 100 blocks, then whatever you do to them or with them, you will always have 100 of them at the end. You can't destroy them, only move them around, change their appearance, or split them up, but there will always be 100 blocks in total. Sometimes you may seem to lose one because it has been changed into a block that can't be seen, but all 100 are still there. 
Perpetual motion, which refers to a device that would continue in motion forever without any additional energy input, violates laws one and two of thermodynamics. According to the first law of thermodynamics, energy cannot be created or destroyed, and a perpetual motion machine would have to produce work energy without any energy input. Second law of thermodynamics The entropy of an isolated system not in equilibrium will tend to increase over time, approaching a maximum value at equilibrium. 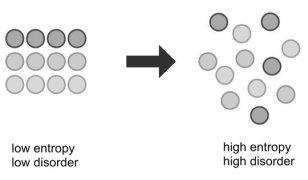
This is possibly the most famous and important laws of all science, and at the same time, the least well understood by those unfamiliar with science. Heat at a given temperature cannot be converted entirely into work; some will escape. Entropy measures the disorder or randomness of a closed system. The universe as a whole is becoming more random and less organized. Note once again that Earth, with its many energy sources, including a massive amount of energy input from the Sun, is NOT a closed system. That's why flowers can grow. More about entropy later. Third law of thermodynamics As the temperature of a system approaches absolute zero, the entropy of a system approaches a constant minimum. The third law is just an absolute reference point for measuring entropy, saying that at absolute zero (-273.15° Celsius or 0° Kelvin), all movement stops. Entropy Entropy is the measure of disorder and randomness in a system. Here are two examples: Letís say you have a container of oxygen molecules. If all the molecules were in one corner, this would be a low entropy state (highly organised). As the molecules move around and fill up the rest of the container, the entropy (disorder) increases. A car at rest has a certain amount of chemical energy stored in its gasoline. This energy is highly organized in one place, with low entropy. As you start the car and begin moving, this chemical energy is converted to work as mechanical energy that moves the driveshaft and wheels. Some of it changes to heat energy that is absorbed by the engine and the road (through friction). This widely dispersed energy is now very disorganized, with a high state of entropy. As entropy increases, less and less of the energy is available to do work. On Earth, we still have great stores of energy, such as fossil, geothermal, and nuclear fuels, as well as energy from the Sun, which also gives us hydroelectric potential energy and wind energy (due to the As these are used, a certain fraction of the energy they contain can never be used to do work, as it escapes as heat loss and various forms of friction. Eventually, all fuels will be exhausted, all temperatures will equalize, and it will be impossible for heat engines to function, or for work to be done. However, this won't happen any time soon; the Sun at least will be around in its present form for another five billion years. But in terms of the universe, and the very long-term, very large-scale picture, the entropy of the universe is increasing, and so the availability of energy to do work is constantly decreasing. Robert Frost described this in his poem Fire and Ice: eventually, when all stars have died, all forms of potential energy have been utilized, and all temperatures have equalized (depending on the mass of the universe, either at a very high temperature following a contraction, or a very low one, following infinite expansion (currently the accepted theory) when all activity ceases there will be no possibility of doing work. Either way, the universe is destined for thermodynamic equilibrium ... maximum entropy. This is often called the heat death of the universe, (when all heat is gone) and will mean the end of all activity. (Confusing name; it implies the universe will end in heat. It really means that all heat will have died out). However, whether the universe contracts and heats up, or continues to expand and cools down, the end is not near. Calculations based on the physics of black holes suggest that entropy can easily continue for at least 100 trillion (1014) years. |