 Air is all around us. It is invisible, but we can feel it when it blows past us, and as we breathe it in. What do you really know about air ... how much does it weigh? what is it made of? can it be used for anything besides breathing? why do we need it to survive? Before we examine what air is, in detail, here are a few fun facts about air. First, a simple question: How much does air weigh? What does one cubic metre of air weigh? What's your guess?Check the answer here. Got it wrong, didn't you!? See a simple experiment that will let you weigh air. Did you know that moist air weighs less than dry air? This is because the number of molecules in a fixed volume of gas is always the same; water vapour molecules in moist air weigh less than the nitrogen and oxygen molecules which they displace. Our Atmosphere: 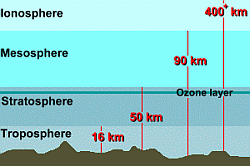 Earth's atmosphere is about 480 km thick, but most of it (about 80%) is within just 16 km of the surface. The upper portion of the atmosphere just gets thinner and thinner, until it merges with outer space. Earth's atmosphere is about 480 km thick, but most of it (about 80%) is within just 16 km of the surface. The upper portion of the atmosphere just gets thinner and thinner, until it merges with outer space.
However, the atmosphere really isn't very thick; in the photo at the right, the blue line around the Earth represents about 450 km, or just about the entire atmosphere! 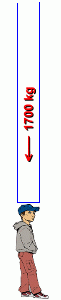
Earth maintains an atmosphere because of its gravitational pull. As a result, most air is found closest to the Earth's surface. Higher up, the air becomes less dense, or thinner. Experienced mountain climbers know this, and carry oxygen tanks to assist breathing at high altitudes ... somewhere above 3 kilometres (the top of Mt. Everest is just over 7.6 km high). Since air is a gas, it is compressible. This means that the air pressure nearer the surface of the Earth is greater than the air pressure in the stratosphere. Air exerts pressure on everyone and everything. Increasing the air pressure makes the air denser. At the Earth's surface, the pressure of all that air above you is 14.7 pounds per square inch, or about 1 kilogram per square centimetre. That doesn't sound like much, but it means the weight of the air on every square foot of surface is is 2117 pounds, or 10335 kilograms on every square metre! That's about 1700 kilograms of weight on your head! When an airplane climbs higher into the atmosphere, the air will be less dense, so it will be easier to fly through. But it will also provide less lift, and there will be less oxygen, so its engines will have to work harder to get enough oxygen to burn. Passengers will suffer from hypoxia (a deficiency of oxygen) if air within the plane is not pressurized. Our atmosphere, which we generally refer to as 'air', is actually a mixture (solution) of many different gases. The composition of the atmosphere is approximately 80% nitrogen and 20% oxygen, with a trace of other gases. Here is the breakdown in more detail: 
Nitrogen: Nitrogen gas (N2) makes up 78% of our atmosphere. Nitrogen is an element which is essential for growth and reproduction in both plants and animals. It is found in amino acids that make up proteins in our bodies, in nucleic acids that comprise our hereditary material called DNA, and in many other organic and inorganic compounds. Atmospheric nitrogen is converted to nitrate (NO3) by lightning. Organic nitrogen compounds are released to the atmosphere when plants decay. Also, industrial pollution and fossil fuel combustion release gaseous nitrous oxides and nitric acid (one component of acid rain). Atmospheric nitrogen in these various compounds makes its way into the oceans and onto land dissolved in rainfall. Oxygen: The gas oxygen (O2) is made up of oxygen molecules, each of which composed of two oxygen atoms. It is colourless, odourless, and tasteless. Oxygen makes up 21% of the air around us, but it also comprises 86% of the oceans and 60% of the human body (since every water molecule contains one oxygen atom), and is the third most abundant element found in the sun, after hydrogen and helium. Almost all plants and all animals require oxygen for respiration to maintain life. When you breathe air in, 21% of that breath is oxygen. Your lungs extract some of that oxygen, and replace it with some carbon dioxide, and so the air you breathe out has a little less oxygen and a little more CO2 in it. (It's important to note that the air you breathe out still contains oxygen ... otherwise mouth-to-mouth resuscitation wouldn't work!) Pure oxygen is very reactive gas, and combines easily with many other subtances to form 'oxides'. A very common oxide is rust, which is what you get when oxygen reacts with the iron in steel. The rate at which oxidation occurs varies with the element with which oxygen is reacting. Rust, or iron oxide, forms relatively slowly. Burning or combustion, however, where oxygen combines with substances in fuel, involves a very rapid oxidation. The carbon in wood, for example, can be quickly oxidized to carbon monoxide and carbon dioxide, with lots of heat given off. In the stratosphere, oxygen molecules combine with free oxygen atoms to form ozone (O3). This ozone gas collects in a thin layer near the top of the stratosphere. Ozone absorbs ultraviolet radiation from the sun, and protects life on Earth from its damaging effects. But there isn't much of it ... if all the ozone in the stratosphere was compressed to ordinary pressure at ground level, it would occupy a layer only 3 mm thick. That's why holes are appearing in the ozone layer due to chemical reactions with pollutants. The sun heats the surface of the Earth unevenly, so that in some places it is warmer, while in other places it is colder. Air closest to the Earth's surface is usually the warmest, and air temperature drops as you go higher. Warmer air close to the surface is less dense, so it rises. Cold air, which is more dense, sinks. As warm air rises, air from cooler areas flows in to take the place of this heated air. This process is called convection and causes air to move, creating wind. A History of Earth's Atmosphere:  Earth's original atmosphere was similar in composition to the nebula of gas from which the planets formed, 5-10 billion years ago. It may have resembled the atmospheres we now see on Saturn, Jupiter, and the other gas giants, which were too massive to lose their original atmospheres to space. Earth and the other inner planets were small enough so that they eventually lost their original atmospheres. Earth's original atmosphere was similar in composition to the nebula of gas from which the planets formed, 5-10 billion years ago. It may have resembled the atmospheres we now see on Saturn, Jupiter, and the other gas giants, which were too massive to lose their original atmospheres to space. Earth and the other inner planets were small enough so that they eventually lost their original atmospheres. Earth's was replaced by compounds outgassed from the cooling crust, such as carbon dioxide, water vapor, sulphur dioxide and nitrogen, or perhaps from impacts of comets rich in gaseous materials. Life forms on Earth have since modified the composition of the atmosphere. In particular, oxygen was first produced by cyanobacteria (blue-green algae) about 2 billion years ago. All the oxygen in Earth's atmosphere is believed to have come from photosynthesis. The vast majority of this oxygen production now occurs in our oceans, by algae and bacteria. Plants and animals use the oxygen and produce carbon dioxide. Without plants, from algae to the trees in the rainforests, there would be no photosynthesis. Without photosynthesis, the oxygen in the atmosphere would be gone in a few thousand years. |